DFW Airport: Cornerstone of North Texas Innovation for 50 Years
|
For 50 years, the DFW Airport has served as a driving force of progress and development in North Texas. The foresight and collaborative spirit of those who came together to establish DFW Airport so many years ago, have helped transform the region into a thriving economic hub. By serving as a nexus for major transportation, DFW Airport has not only fueled local economies but has also attracted global visitors and facilitated the influx of residents and businesses, thus defining the identity of Dallas and Fort Worth.
From hosting iconic events like the Concorde's inaugural landing in the United States to welcoming dignitaries such as the NASA space shuttle Atlantis and Air Force One, DFW Airport has consistently been on the world stage as a premier aviation hub.
Continuing a tradition of innovation and progress, DFW Airport is embarking on modern upgrades, including a commitment to carbon neutrality by 2030, and the addition of new terminals. DFW Airport is poised to continue its legacy of excellence and serve as a catalyst for growth and connectivity for years to come.
|
National Science Foundation Expands Funding Opportunity
to Bolster Regional Innovation Engines
|
The U.S. National Science Foundation announced a funding opportunity to invest in a new set of NSF Regional Innovation Engines (NSF Engines) across the U.S. In January, NSF announced the 10 inaugural NSF Engines, and the latest opportunity looks to build upon that investment, pending congressional appropriations.
The initial NSF Engines announcement represented one of the single largest broad investments in place-based research and development in the nation's history — uniquely placing science and technology leadership as the central driver for regional economic competitiveness. This latest funding opportunity advances the bipartisan priorities outlined in the "CHIPS and Science Act of 2022," which authorized the NSF Engines program.
|
Navigating Clinical Trials & Maximizing Innovation
in Drug Therapy Development
|
Join BioNTX and Caidya for an engaging, in-depth exploration of the clinical trial landscape and the necessary steps to ensure successful outcomes.
Caidya's team of dedicated experts will discuss effective strategies to streamline early phase trial design and address CMC challenges head-on. Founders and innovators, come learn how to better optimize efficiency while fostering innovation in your pipelines.
|
After the program, relax and unwind with peers, founders, and innovators at our May BIO BREAK Happy Hour sponsored by Caidya. Forge genuine connections for professional business growth, and experience the vibrant DFW Life Science ecosystem.
|
UT Tyler School of Medicine Receives Anonymous Gift
to Fund Hospice & Palliative Care Fellowship
|
The University of Texas at Tyler School of Medicine announces it has received a $400,000 gift from an anonymous donor to support the hospice and palliative medicine fellowship and retain physicians in the East Texas region. “This generous donation to our hospice and palliative medicine fellowship program signifies a significant step forward in our mission to provide exceptional health care services to the East Texas community,” said Dr. Brigham C. Willis, School of Medicine founding dean. “In particular, education on chronic disease management, end of life issues, quality of life improvement and so much more, as is provided in this fellowship, is fundamental to our mission of training well-rounded, humanistic physicians. This support will not only benefit our fellows but also the patients and families they serve.”
|
Nanoscope Therapeutics Draws Arlington Policy Visit
from Local, State and Federal Representatives
|
This week, Nanoscope Therapeutics hosted a visit from Congressman Jake Ellzey, Arlington City Council Members, Arlington Economic Development, BIO Government Relations, and other guests for a presentation about Nanoscope’s groundbreaking work, and a tour of the company's Arlington facility.
Co-founder and president, Dr. Samarendra Mohanty discussed the science behind Nanoscope’s lead program MCO-010, a mutation-agnostic gene therapy for patients with permanent and severe vision loss from advanced retinitis pigmentosa (RP). MCO-010 has received Orphan Drug and Fast Track designation by the FDA after recently announcing Phase 2b clinical trial results “demonstrating a statistically significant improvement of best-corrected visual acuity” in patients with advanced RP.
Dr. Mohanty and Sulagna Bhattacharya, Nanoscope co-founder and CEO, also shared the potential growth they foresee in North Texas that will contribute to Dallas-Fort Worth becoming another key hub in the country’s biotech ecosystem. They also discussed the critical importance of legislative issues such as the Orphan Cures Act, which fixes a flaw in how the Inflation Reduction Act impacts research into rare disease drugs, and full R&D expensing, which supports investment in rare drug research that can take years to pay off.
|
Island Pharmaceuticals CEO Calls for Continued Advancements
in Dengue Prevention & Therapy
|
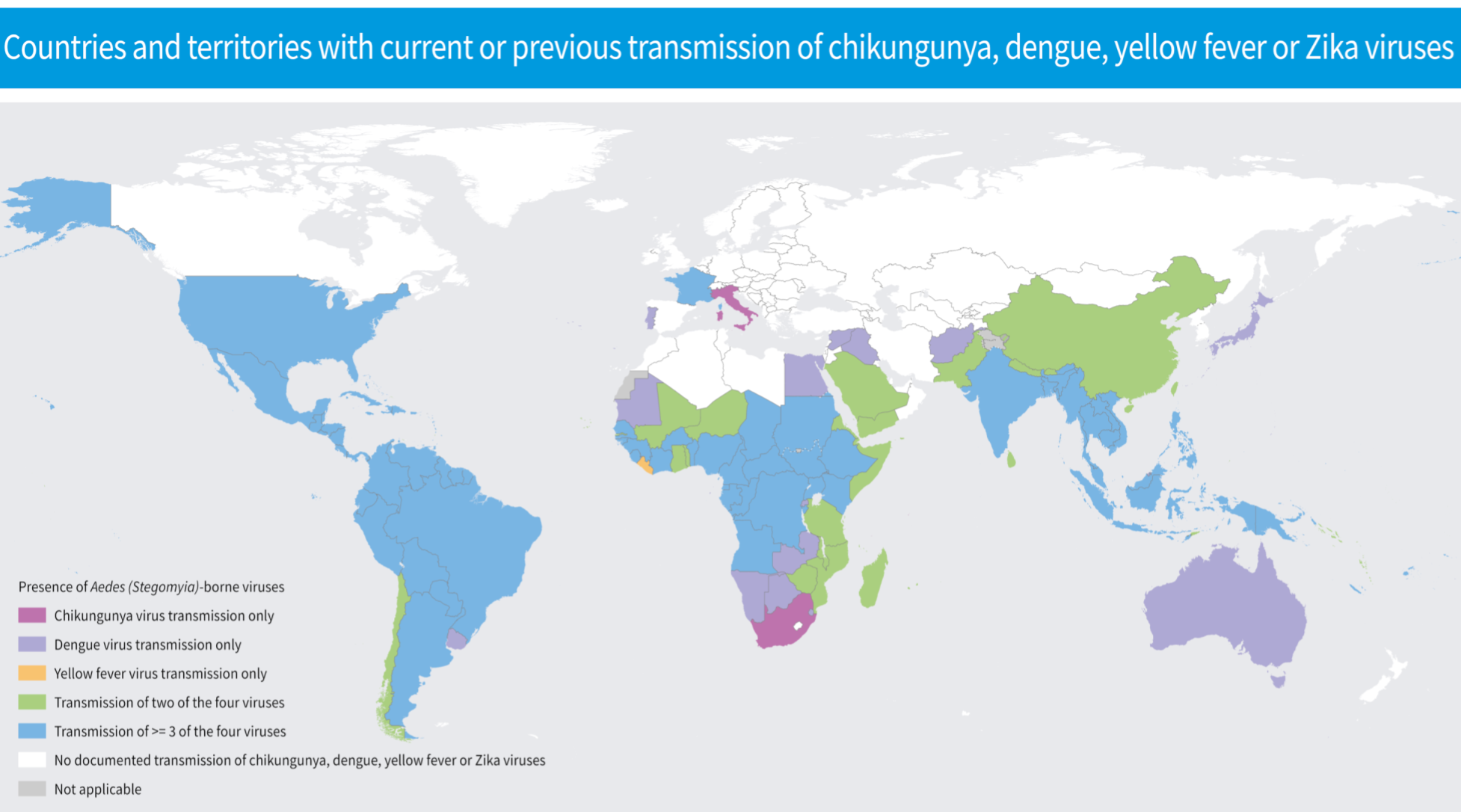
(above: map of global arbovirus distribution) Arthropod-borne viruses (arboviruses) such as dengue, yellow fever, chikungunya and Zika viruses are all current public health threats in tropical and sub-tropical areas where approximately 3.9 billion people live. The frequency and magnitude of outbreaks of these arboviruses, particularly those transmitted by Aedes mosquitoes, are increasing globally, fueled by the convergence of ecologic, economic and social factors.
|
Island Pharmaceuticals CEO Dr. David Foster recently shared exciting updates on their progress in developing a treatment for dengue fever. Highlighting promising results from a recent single ascending dose study of ISLA-101, Foster emphasized the encouraging significance of the company's newly-assessed pharmacokinetics data. The findings support the urgent need for effective treatments for dengue fever, which affects around 400 million people annually around the globe.
|
European Commission Approves Johnson & Johnson's Cilta-Cel
for Treatment of Multiple Myeloma
|
Janssen-Cilag International NV, a subsidiary of Johnson & Johnson, announces they have received European Commission approval for CARVYKTI® (ciltacabtagene autoleucel; cilta-cel) to address relapsed and refractory multiple myeloma in adults who have undergone prior treatment, including lenalidomide, and have experienced disease progression. This milestone makes cilta-cel the first BCMA CAR-T therapy approved in Europe for eligible patients at the first relapse stage, following encouraging results from the Phase 3 CARTITUDE-4 study, which demonstrated its efficacy and safety.
|
NIH Announces Sweeping Pay Increase for Researchers
at Grantee Institutions Across
|
The National Institutes of Health (NIH) announced it will increase annual pay levels for scholars at NIH-funded external institutions who are recipients of the Ruth L. Kirschstein National Research Service Awards (NRSA).
The increase applies to more than 17,000 research trainees and includes additional funds for childcare and training-related expenses. Pending the availability of funds through future appropriations, the NIH intends to further increase stipend funding levels over the next three to five years to reach a starting pay level of $70,000 annually for postdoctoral NRSAs. Additionally, NIH-funded institutions may supplement NRSA recipients’ new pay levels with additional, non-NIH funds and/or benefits.
|
Lantern Pharma Launches Recurring Webinar Series
on Heels of Clinical Trial Expansion to Japan & Taiwan
|
In a press release double-header, Lantern Pharma announced the expansion of its Harmonic™ trial for LP-300, targeting non-small cell lung cancer (NSCLC) in never-smokers, to include Japan and Taiwan. Dr. Yashushi Goto of the National Cancer Center of Japan will lead the trial in Japan. This expansion aims to accelerate data collection for LP-300's evaluation as a therapy for relapsed and inoperable primary adenocarcinoma of the lung when combined with chemotherapy, providing a crucial treatment option for never-smoking lung cancer patients in these regions.
Following news of the Harmonic™ trial expansion, the company publicized the upcoming launch of a recurring series, “Webinar Wednesdays” to feature world-class physician scientists & key opinion leaders discussing critical areas of oncology drug development.
|
The Path to Affordable Medication: Cost Plus Drugs
Announces Partnership Expansion with Vivid Clear Rx
|
The partnership between Cost Plus Drugs and Vivid Clear Rx aims to revolutionize access to affordable prescriptions for patients. Eligible members of Vivid Clear Rx can now easily purchase medications directly from Cost Plus Drugs using their pharmacy benefit plan, streamlining the process. Both entities are committed to transparent pricing: Cost Plus Drugs offers medications at cost plus a 15% markup, while Vivid Clear Rx adopts a 100% pass-through approach. This collaboration is poised to alleviate the burden of rising prescription costs for health plans and patients nationwide, ensuring clarity and affordability in medication expenses.
|
EisnerAmper Receives '2024 Best Company to Work for' Award
|
EisnerAmper, a global accounting firm, has been named the "2024 Best Company to Work for in New York" in the large company category by The NY State Council of the Society for Human Resource Management and the Rochester Business Journal. Chief Human Resources Officer, Maureen Paradine, expressed gratitude for the recognition, emphasizing the firm's commitment to both clients and employees, fostering an atmosphere of appreciation and collaboration.
|
Perkins&Will Launch San Antonio Studio to Meet Texas Demand
|
The San Antonio studio: Adrianna Swindle (fourth from left), principal of corporate and commercial practices, and Omar Cantu (seated, right), health practice leader.
|
Design firm Perkins&Will, renowned for its innovative architecture and design solutions, has announced the opening of a new studio in San Antonio, Texas. This strategic expansion reflects the growing demand for the firm's services in the U.S. Southwest region. With established studios in Dallas, Austin, and Houston, the move cements the firm's commitment to delivering exceptional vision and design expertise across Texas and the life sciences.
|
RSM Leaders Recognized in
Forbes' Top 200 CPAs
|
RSM US LLP, a leading provider of assurance, tax, and consulting services for the middle market, proudly celebrates the inclusion of Managing Partner & CEO Brian Becker and three other esteemed partners—Laura Dietzel, Sara Lord, and Kurt Shenk—in Forbes' inaugural Top 200 CPAs list. This prestigious recognition underscores their exceptional track records, innovative leadership, and significant contributions to both the profession and their communities. Selected through independent nominations and endorsed by esteemed societies and associations of CPAs, these individuals exemplify expertise, innovation, and integrity in public practice, reflecting a steadfast commitment to excellence and service in their respective fields.
|
Baylor University Scientist Receives Prestigious NSF Award
for Animal Behavior Research
|
Dr. Sarah Kienle, an esteemed assistant professor of biology at Baylor University, has been honored with a Career Development Award from the National Science Foundation’s Faculty Early Career Development (CAREER) Program. With a focus on animal behavior, particularly in leopard seals, Dr. Kienle's research investigates the complex dynamics of female-biased dimorphism in these apex predators of the Southern Ocean.
|
The Program is an NSF-wide initiative that offers the Foundation's most prestigious awards in support of early-career faculty who have the potential to serve as academic role models in research and education and to lead advances in their department or organization. This NSF program especially encourages women, members of underrepresented minority groups, and persons with disabilities to apply.
|
Revolutionizing Diabetic Foot Care
Through Advancements in Insole Technology
|
UTARI researchers Veysel Erel and Aida Nasirian with their diabetic shoe technology
|
The UT Arlington Research Institute (UTARI) has developed an innovative shoe insole technology led by research scientist Muthu B.J. Wijesundara. Wijeseundara developed the technology to reduce the risk of diabetic foot ulcers. Unlike traditional insoles, UTARI's pressure-alternating design cyclically relieves pressure from different foot areas, enhancing blood flow and tissue rest.
About one-third of people with diabetes develop foot ulcers during their lifetime. In the U.S., more than 160,000 lower extremity amputations are performed annually due to complications from diabetic foot ulcers, costing the American health system about $30 billion a year.
|
Advancing Respiratory Health Monitoring: A Breakthrough Algorithm for Early Detection
|
A University of Texas at Dallas researcher- together with international colleagues, have developed an algorithm to detect respiratory issues like asthma attacks, by analyzing wheezing frequency in real-time breathing data. Led by Dr. Dohyeong Kim, the team includes physicians, environmental scientists, engineers, and AI technicians from South Korea.
Published in PLOS ONE, the algorithm offers a significant advancement in respiratory health monitoring, potentially integrating into wearable devices for automatic alerts to patients or caregivers, allowing prompt intervention and improving health outcomes amidst global respiratory disease burdens.
|
Legacy of Excellence: Advancing Osteopathic Medical Education
|
Dr. Lisa Nash, recognized with the 2024 Special Lifetime Achievement Award from the Assembly of Osteopathic Graduate Medical Educators, has led transformative initiatives in osteopathic medicine and graduate medical education. Serving at The University of North Texas Health Science Center at Fort Worth’s Texas College of Osteopathic Medicine, her visionary leadership and unwavering commitment have advanced graduate medical education, fostering excellence and innovation. This award acknowledges Dr. Nash's remarkable contributions, shaping the future of osteopathic medicine.
|
UT Southwestern Supercomputer Begins Running Simulations Unlocking the Secrets of Membrane Fusion
|
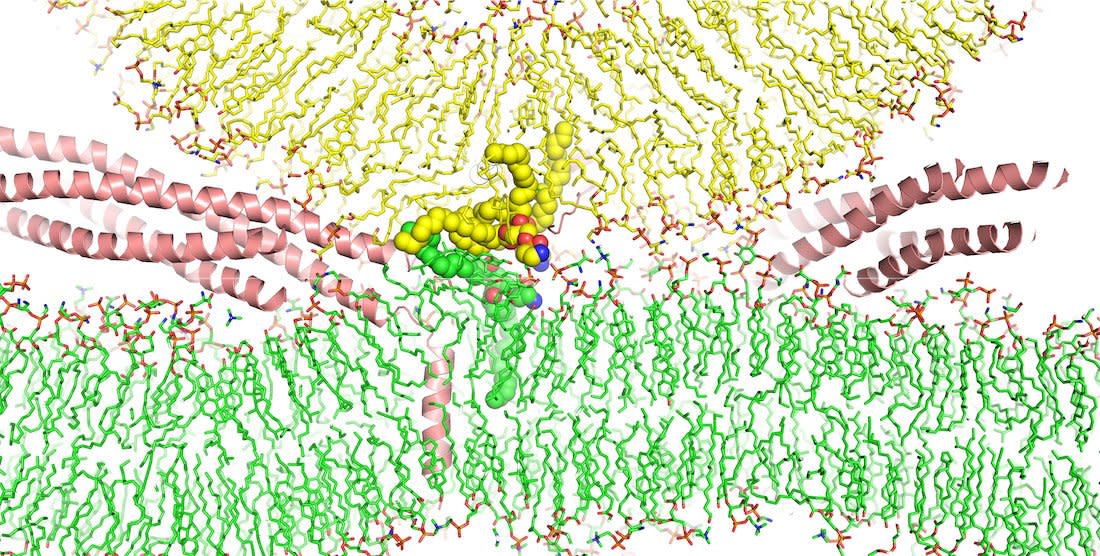
An image shows how SNARE proteins (in salmon color) initiate the fusion of two membranes by facilitating encounters of their water-repelling tails (yellow and green) at the water-filled interface between the membranes (illustrated by the tails shown as spheres).
|
UT Southwestern Medical Center researchers utilized a powerful supercomputer to investigate how SNARE proteins facilitate membrane fusion. Led by Jose Rizo-Rey, Ph.D., their study challenges previous assumptions, revealing that membranes brought into contact typically do not fuse. This breakthrough enhances our understanding of membrane fusion and offers potential for innovative treatments for related conditions.
|
Federal R&D Funding Webinar Sheds Light on Diverse Pathways
|
The passcode to access the webinar is F8agKt?1.
|
Dr. Travis Taylor, PhD from SMI hosted an engaging educational webinar elucidating the nuanced journey to securing government funding. The event also featured Memsel CEO, Thomas England, bringing personal experience from a large, recent appropriation and Lito Rodriguez, Grant Specialist with over 20 years of experience in helping large institutions and startups secure government research funding.
Attendees were quick to take notes as speakers walked through classic and innovative strategies to progress in various funding scenarios. Speakers also explored the differences in company profiles for smaller nondilutive funds such as SBIR and NIH grants to securing larger awards for advancing R&D.
|
Form Bio Recognized for Cell and Gene Therapy Advancement with 2024 Excellence Award
|
Form Bio has been announced as the winner of the Excellence Award in Cell and Gene Therapy Advancement 2024 from Global Health and Pharma (GHP) for their Cell and Gene therapy software and AI solutions. Form Bio's platform accelerates timelines between discovery to clinic using rapid in silico to characterize, analyze, compare, and optimize drug candidates.
Form Bio continues to be a leader in AI for drug discovery with development programs dedicated to Drug Candidate Design, Testing and Selecting, Test Manufacturing, Drug Validation, and IND enabling studies.
|
Improving Patient Outcomes Through Medical Device Innovation
|
Founded in 2021, Design 33 is an innovative medical device company developing cutting-edge, transformative medical technologies ranging from implantable devices in the domain of Interventional Radiology to multiple, distinct specialty needles. Leveraging more than twenty-five years of design and product development expertise founder and principal, Simon Forber, has made it his life’s mission to develop unique medical device solutions and identify opportunities where current products do not adequately address user and patient needs.
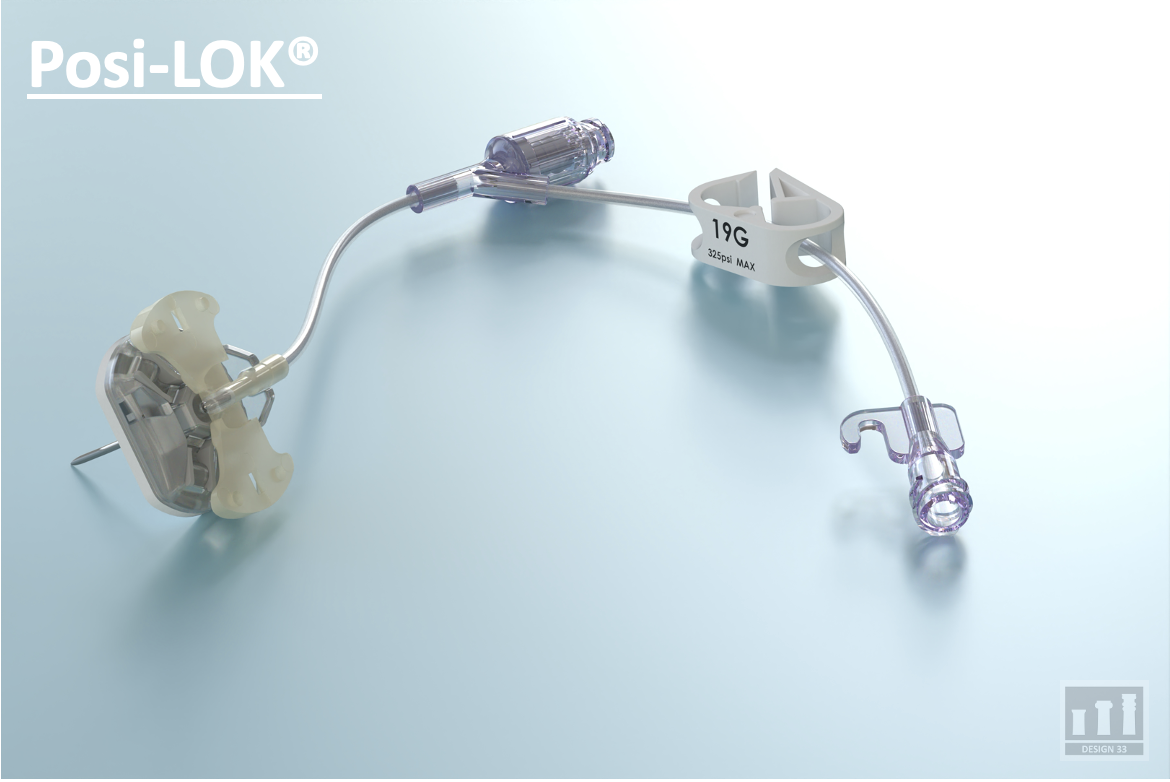
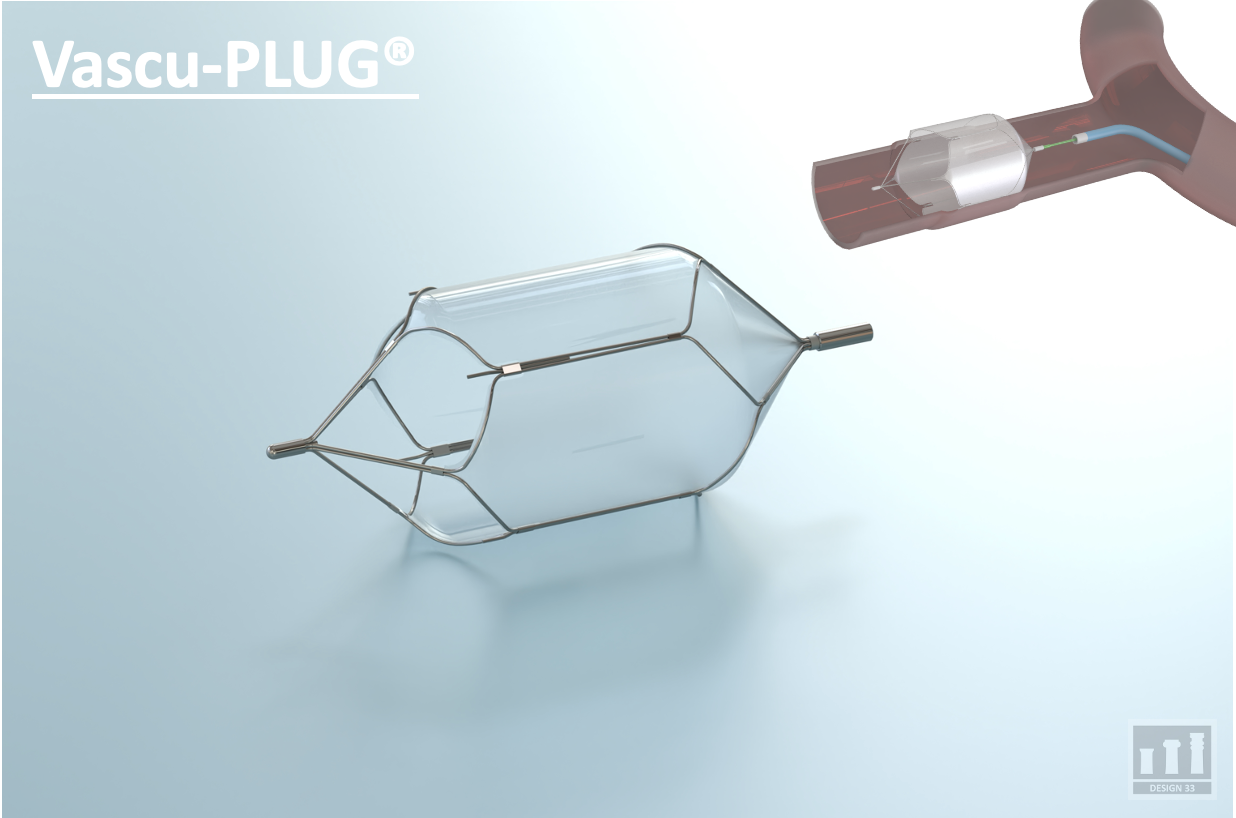
Currently, Design33 has two projects in development. The first, a Safety Huber Needle (Posi-LOK) addresses the clinical need for reducing catheter occlusion risks in implantable venous ports. The company's second device, a game-changing vascular plug (Vascu-PLUG) for Interventional Embolization. Design 33 has forged a partnership with a major US company to commercialize the device with the goal of entering the market in 2025.
|
Health Wildcatters' WISH Network Takes Top Honors
in 2024 Growth Accelerator Fund Competition
|
Members of the Women in Science and Healthcare (WISH) initiative at Pegasus Park
|
Health Wildcatters won the First Stage of the U.S. Small Business Administration’s 2024 Growth Accelerator Fund Competition (GAFC) for their “Women in Science and Healthcare” (WISH) initiative this past week. The initiative earned itself a cash prize for fostering an impactful and inclusive approach to a collaborative an innovative ecosystem for advancing small business R&D.
The WISH Network boasts over 250 people regularly engaging to support female entrepreneurs. With this prize, they will be launching a pilot program to expand to university partners in the North Texas region.
|
FUNDING & MENTORSHIP OPPORTUNITIES
|
CPRIT Funding Programs Drive Cancer Research
Product Development & Prevention
|
Through these funding programs and opportunities, CPRIT grows the cancer-fighting ecosystem across Texas, providing life-extending results, and tangible economic benefits throughout the State.
|
SEED Awards
This award funds early-stage development of oncology therapeutics, devices, diagnostics, or tools, aiming to advance promising technologies toward commercial viability. Applicants should have identified a novel technology, conducted preliminary safety testing, demonstrated manufacturing feasibility, assessed the business opportunity, and established a company.
|
Texas Diagnostic and Devices Company Awards
This award covers areas such as cancer detection, diagnosis, prognosis, and monitoring, including companion diagnostics. Applicants should have developed commercial prototypes or assays validated for human samples. Funding supports preclinical proof of concept, product validation, manufacturing, and clinical studies to demonstrate safety and efficacy.
|
Texas New Technologies
Company Awards
This award supports ongoing research and development of new cancer detection, diagnosis, prognosis, monitoring, or treatment technologies. Created by CPRIT, it funds projects that don't fit into other CPRIT Product Development Research RFAs. Proposals may include bioinformatics, artificial intelligence, radionuclide production, cell-based therapy manufacture, sample quality improvement processes, and therapeutic biomanufacturing. Companies may use CPRIT funds for preclinical proof of concept, product validation, design, manufacturing, development, and clinical studies with appropriate justification.
|
Texas Therapeutics Company Awards
This award funds ongoing research and development of innovative products, services, and infrastructure with significant potential for patient care. Typically, companies at this stage have identified lead compounds, demonstrated efficacy in animal models, completed pilot toxicology studies, established scalable manufacturing processes, and identified prototype formulations. Funding supports preclinical proof of concept studies, manufacturing development, GLP safety studies for INDs, Phase 1 trials for safety and dose determination, and Phase 2 studies for safety and efficacy evaluation in initial patient populations.
|
The Hill Prizes
Recognizing Life Science Innovators & High-Impact Research
Applications open May 1, 2024
|
Generously funded by Lyda Hill Philanthropies, the Hill Prizes aim to accelerate groundbreaking research ideas with high potential for real-world impact. These prizes celebrate the achievements of top innovators and researchers in Texas, spanning six categories: Medicine, Public Health, Engineering, Biological Sciences, Physical Sciences, and Technology. Each recipient of the prize will be awarded $500,000 in funding to further their work and drive innovation.
|
PLMC 2024 Introduces Poster Presentations to Highlight Innovative Pharma Logistics Research
Abstracts due June 1, 2024
|
PLMC 2024 is thrilled to unveil Poster Presentations as a new highlight of the event, providing a platform for researchers to showcase innovative studies in Pharma Logistics. Led by Prof. Roel Gevaers from the University of Antwerp, this addition aims to foster collaboration and knowledge exchange among industry professionals and academics. Researchers from academia and industry are encouraged to submit abstracts covering topics such as pharmaceutical supply chain optimization, economic implications, innovative engineering solutions, regulatory challenges, sustainability considerations, technological advancements, risk management, patient-centric approaches, and data analytics in pharmaceutical distribution. This initiative promises to enrich dialogue and contribute to advancements in the field of pharmaceutical logistics.
|
Chiesi Rare Disease Golden Ticket Competition
Applications due June 30, 2024
|
The Chiesi Golden Ticket Competition awards startups and companies 2 (two) Golden Tickets to a US BioLabs site. Each Golden Ticket provides one year of fully paid individual bench space and membership at a fully equipped laboratory designed to accelerate early-stage startup working on research and development in the gene editing and/or relevant viral and non-viral delivery technologies, for treating rare hematology, immunology, ophthalmology, dermatology, inborn errors of metabolism or metabolic diseases.
|
Upcoming Events & Opportunities
|
RNA Biology and Therapy Symposium
Join the RNA Biology and Therapeutics communities at UTSW to explore new opportunities for collaborative basic and translational RNA-focused science. Talks and posters from faculty, trainees, and staff will be presented.
April 30, 2024 | Dallas, TX
|
Safe & Secure Biotechnology Platforms
Join the ARPA-H CX Hub to learn more about the Network Survey for Safe & Secure Biotechnology Platforms and toolkits that aim to leverage information developed through the APECx Program.
May 2, 2024 | Dallas, TX
|
The Science of Socializing
Join Azzur Group for an evening of networking, where you can enjoy complimentary food and beverages, engage in meaningful conversations, and build valuable connections with like-minded individuals in the Life Sciences community.
May 2, 2024 | Dallas, TX
|
Convergence AI Dallas
Join Dallas Regional Chamber to hear cutting-edge trends and innovations in artificial intelligence (AI) and its potential for revolutionizing business practices.
May 2, 2024 | Irving, TX
|
2024 Ignite Golden Ticket
Join Ignite Healthcare to hear women leading digital health and medical device startups to win The Golden Ticket to participate in their one-of-a-kind Accelerator Program.
May 2, 2024 | Dallas, TX
|
FWLSC's 2024 CRISPR Update
Join Fort Worth Life Sciences Coalition for great music, delicious snacks and desserts, an interesting, informative program, and edifying conversations about CRISPR.
May 2, 2024 | Fort Worth, TX
|
Nucleate Texas Activator Program
Join Nucleate Texas to hear students talk about their novel and innovative solutions to today’s most challenging issues in life sciences for a chance to win a Golden Ticket at Biolabs!
May 7, 2024 | Dallas, TX
|
BIO BREAK
Join McDermott, Deloitte, and BioNTX for cocktails at May's BIO BREAK. Relax, engage with the life science community, and cultivate genuine connections that open doors to professional growth.
May 9, 2024 | Dallas, TX
|
The iC³ Life Science Summit: 10 Years of Innovation
October 3, 2024 - October 4, 2024
|
This year's 10th annual BioNTX iC³ Life Science Summit will provide dynamic platform and a gorgeous setting for the North Texas bioscience community to share insightful discussion, cutting-edge research, and emerging technologies set to revolutionize the future of healthcare and biomedicine. With a new, international focus; the 2024 iC³ Life Science Summit is proud to welcome the global life science community to North Texas.
|
Tech Transfer Showcase Applications Open in May!
|
Applications for the BioNTX University Tech Transfer Showcase opening soon!
The Tech Transfer Showcase at the iC³ Life Science Summit provides founders with an opportunity to present innovative intellectual property to a select audience of investors and industry leaders, opening doors to potential collaborations and funding opportunities. Become part of the growing legacy of North Texas Life Sciences and apply to present your company!
|
Renew Your Membership in 2024
Our members are the Foundation of our work and the driving force behind industry growth in North Texas. BioNTX provides members with opportunities to connect and collaborate to gain business insights, thought leadership, access vital resources, share innovations, and increase market visibility.
|
|