|
|
NORTH TEXAS POLICY INTERESTS |
|
Vote in November 2025 — Advance Dementia & Brain Health Research |
|
|
Brain health touches nearly every Texas family and community. From Alzheimer’s and PTSD to depression, addiction, and traumatic brain injury, these challenges shape lives, strain families, and impact the state’s workforce and economy. According to recent findings from The Perryman Group, the annual cost of untreated or under-addressed mental and brain health conditions exceeds $400 billion in lost spending, productivity, and earnings across Texas.
As you prepare to cast your ballot, we encourage you to explore Proposition 14, understand its implications, and share what you learn with your networks. Inform yourself—and then decide
By staying informed and engaged, we can elevate the conversation about brain health in Texas—and help ensure that future generations benefit from innovation, research, and a state committed to progress. |
|
GROUNDBREAKING NEWS |
|
BioNTX and IISc Foundation Forge Global Life Sciences Partnership |
|
|
|
|
Left Photo: Eric B. Moore of BioNTX, and Chandini Kumar Portteus, MPH
Right Photo: Rebecca Wong, MPH, MBA, George Brody, MScE, MScCS of IISc Foundation, Usha Vijayraghavan, PhD of IISc, Sundar Swaminathan, MD, DM, ABIM of IISc, Navakanta Bhat, PhD of IISc, Govindan Rangarajan, MSc, PhD of IISc, Sanjeeva Kalva, MBBS, MD of UT Southwestern Medical Center and Chandini Kumar Portteus, MPH
|
|
BioNTX, the North Texas bioscience and healthcare innovation network, has signed a strategic Memorandum of Understanding (MOU) with the Indian Institute of Science Foundation (IISc Foundation). The partnership creates a cross-continental bridge to support clinical research, translational science, and global healthcare innovation.
The collaboration formalizes a framework for long-term ties between the Indian and North Texas life sciences ecosystems, fostering translational research, medical education, and affordable healthcare solutions. Both institutions share a commitment to advancing science, equity, and innovation to improve health outcomes worldwide. |
|
COMING SOON |
|
BIO BREAK is Back and Expanding |
|
|
BIO BREAK is expanding—continuing in Dallas, Fort Worth, and now adding Frisco—offering more opportunities to connect, share ideas, and spark collaboration across our ecosystem.
With the momentum of the iC³ Summit carrying us forward, let’s finish the year strong and keep building the relationships that drive life science and healthcare innovation in North Texas. |
|
From Lab to Launch: Navigating Facilities in Life Sciences October 15, 2025 |
|
|
Presents |
|
|
Join the BioNTX Facilities Committee to hear a discussion on guiding small companies through the critical transition from research to production. We will dive into the different paths companies can take to scale their operations, including utilizing co-working facilities such as BioLabs and Bridge Labs, collaborating with contract development and manufacturing organizations (CDMOs), or taking on the challenge of building your own facility from the ground up or through strategic renovations. |
|
MEMBER SPOTLIGHT |
|
Biobridges Accelerates Growth and Innovation Across Life Sciences |
|
|
BioBridges, a leading life sciences consulting and solutions firm, continues to expand its impact across the industry with new leadership appointments, strategic acquisitions, and the launch of its dedicated MedTech division. The company now offers comprehensive Health Economics and Outcomes Research (HEOR) services, broadening its ability to support clients through every stage of product development and commercialization.
Recognized as a top life science consulting provider in 2025, BioBridges continues to grow its national footprint from its new headquarters in the Raleigh-Durham area. The company’s integrated service model—combining expertise across biotech, pharma, and MedTech—has enabled more than 4,000 successful client engagements worldwide. By connecting scientific talent with transformative projects, BioBridges remains focused on advancing innovation and improving outcomes across the life sciences ecosystem. |
|
MEMBER NEWS |
|
Alloy Therapeutics Partners with UBC to Accelerate Antibody Therapeutics for Pandemic Preparedness |
|
|
Alloy Therapeutics and the University of British Columbia have announced a collaboration to advance the discovery of new antibody therapeutics for pandemic preparedness.
Alloy will provide its expertise in antibody discovery to support UBC researchers, who will use high throughput cryo electron microscopy through the PROGENITER program within Canada’s Immuno Engineering and Biomanufacturing Hub. The partnership aims to accelerate the development of innovative therapies for infectious disease targets and strengthen future pandemic readiness. |
|
|
Colossal Biosciences Marks First-Year Milestone in Dire Wolf Research |
|
|
|
Romulus and Remus, two male dire wolves born through Colossal Biosciences genetic engineering advances, have reached their first birthday. The Dallas-based biotechnology company announced in April 2025 that two dire wolf litters had been born: Romulus and Remus in October 2024 and a female, Khaleesi, in January 2025.
Dire wolves once roamed North America alongside saber-toothed tigers and mastodons before going extinct about 13,000 years ago. Colossal researchers used DNA recovered from a 72,000-year-old skull and a 13,000-year-old tooth to recreate the dire wolf genome. |
|
Island Pharmaceuticals Advances Galidesivir Program with FDA Type C Meeting |
|
|
Island Pharmaceuticals Ltd announced that the U.S. FDA has granted a Type C meeting request under Galidesivir’s open IND application to discuss using the Animal Rule to accelerate approval for treating the Marburg virus. The FDA will provide written feedback by November 12, 2025. This marks a key milestone as Island strengthens its role in developing antiviral solutions for high-priority public health threats. |
|
Lantern Pharma Highlights Breakthrough Results for Tumor-Targeted LP-184 |
|
|
Lantern Pharma (NASDAQ: LTRN) is advancing LP-184, an AI-discovered drug candidate with a tumor-activated mechanism that targets cancer cells while sparing healthy tissue.
In The Watchlist, CEO Panna Sharma highlights the strong synergy between LP-184 and PARP inhibitors, which has achieved 100% tumor regression in preclinical models of Triple-Negative Breast Cancer. Powered by Lantern’s RADR AI platform, this approach is accelerating drug development and improving the efficiency and success of clinical trials. |
|
ParaNano Wound Care Advances Infection Monitoring for Chronic Wounds |
|
|
|
(from left to right) JP Jeffries, Chelsea Luxen, Derby Whitefield and Reese Huhnke BioNTX Congratulates ParaNano as one our 2025 Rising Stars! |
|
Oklahoma-based ParaNano Wound Care has developed a nanotechnology solution to continuously monitor chronic wounds for infection, aiming to prevent complications such as sepsis and amputations.
CEO and co-founder Chelsea Luxen said the company plans to reach full market penetration next year, starting with a business-to-business launch before expanding access to all patients. The preclinical company is advancing its WoundCue™ product line with Bio-Z™ Technology and holds exclusive worldwide patent rights from the University of Central Oklahoma and the University of Manitoba. |
|
Secretome Therapeutics and RoosterBio Achieve Commercial-Scale Production of STM-01 |
|
|
Secretome Therapeutics, a clinic-stage company developing neonatal cardiac progenitor cell (nCPC) therapies, and RoosterBio have completed commercial-scale production of STM-01. The 50L bioreactor process replaces the previous 2D flask method, enabling scalable manufacturing for ongoing heart failure clinical trials. The partnership also covers Secretome’s preclinical asset, STM-21, for neurological and inflammatory conditions, using a similar 50L bioreactor process. |
|
Spark Biomedical Awarded Wellcome Leap Funding for Women’s Health |
|
|
Spark Biomedical, parent company of OhmBody, has been selected as one of 13 global recipients of Wellcome Leap’s The Missed Vital Sign initiative. The funding supports its work on heavy menstrual bleeding (HMB) using the non-invasive transcutaneous auricular neurostimulation (tAN) platform, which enhances platelet function and reduces bleeding. The grant will enable a nationwide clinical trial assessing tAN’s safety and efficacy, including in women with von Willebrand Disease (VWD). |
|
TriCelX Announces FDA Registration of Cell Manufacturing Facility in Frisco |
|
|
|
TriCelX, a global leader in evidence-based biologics, announced FDA registration of its ISO Level 7 cleanroom and cell processing facility at The Baylor Scott & White Sports Therapy and Research Center at The Star in Frisco, Texas.
The facility provides a compliant platform for cell therapy manufacturing, supporting safe, high-quality biologics for patients worldwide, including umbilical cord-derived Mesenchymal Signaling Cells (UC-MSCs) for tissue regeneration, neuro-inflammation reduction, and immune modulation. |
|
Deloitte Digital Launches Agentic Patient Services for Life Sciences |
|
|
Deloitte Digital and Salesforce have introduced the Agentic Patient Services suite, AI agents built on Agentforce to streamline patient onboarding, adherence, and care management. The solution improves efficiency, personalization, and outcomes while freeing human teams for meaningful interactions. The Intake Agent will be showcased at Dreamforce 2025 and available on AgentExchange this fall. |
|
Medical Device Security Care |
|
|
The rise of internet-connected medical devices is transforming cybersecurity in health and life sciences. With the smart device market expected to exceed $125.5 billion by 2033, organizations face growing risks from legacy systems, third-party components, and expanded remote care models.
This surge in connectivity brings innovation — but also greater exposure to operational, financial, and patient safety threats. It’s time for health and life sciences leaders to recognize these risks and adopt a “secure by design” approach to protect patients and data alike. |
|
Donate Blood with Carter BloodCare and Save Lives |
|
|
This October, Carter BloodCare urges eligible donors to give blood or platelets to help breast cancer patients and others fighting life threatening conditions.
Treatments like surgery, chemotherapy, and radiation often require transfusions to replace lost blood and platelets. Every donation directly supports patient survival and recovery. Your gift can be lifesaving. Donate today. |
|
|
IDT and Hamilton Launch Automated Solutions for Genomic Research |
|
|
Integrated DNA Technologies (IDT) and Hamilton have partnered to automate comprehensive genomic profiling (CGP) workflows. The collaboration combines IDT’s xGen™ and Archer™ NGS products with Hamilton’s Microlab® STAR™ and NIMBUS® platforms, providing flexible, scalable, and efficient NGS solutions from sample to answer.
The partnership accelerates genomic discoveries, reduces manual workflow steps, and enables reliable biomarker identification, expanding IDT’s sample-to-report offerings for solid tumor and blood cancer research. |
|
|
Munck Wilson Mandala Appoints Merritt D. Westcott to Lead Life Sciences Practice |
|
|
|
Merritt D. Westcott, JD, MS |
|
Munck Wilson Mandala has appointed Merritt D. Westcott, a nationally recognized biotech patent litigator with a background in genetics and molecular biology, to lead its Life Sciences Practice Group. With more than 25 years of experience, Westcott brings deep scientific and legal expertise to guide the firm’s expanding work in biotechnology, MedTech, and digital health.
Under her leadership, the firm will continue expanding its capabilities across biotechnology, digital health, and AI-driven innovation. |
|
Polsinelli Recognized for Advancing Women & Diversity in Law |
|
|
Law360 has ranked Polsinelli #55 in its 2025 Women in Law Report among firms with 600 or more attorneys, up 20 spots from last year. The report benchmarks women attorney representation across all levels, including partnership, highlighting Polsinelli’s commitment to equitable career advancement.
The firm also rose nine spots in Law360’s annual Diversity Snapshot, tracking representation of attorneys of color across seniority levels. These gains reflect Polsinelli’s ongoing efforts to foster an inclusive workplace and ensure diverse perspectives shape the future of the firm and the legal industry. |
|
UTA Receives NIH Funding to Advance Brain Disorder Research |
|
|
|
The University of Texas at Arlington is advancing research on brain development and disorders using neuroimaging and artificial intelligence.
Projects focus on PTSD, depression, anxiety, obsessive-compulsive disorder, and the impact of maternal diabetes on early brain development. By combining global brain imaging datasets with UTA’s MRI facility, researchers are identifying biomarkers to improve diagnosis, treatment, and personalized care. |
|
UTARI Researchers Develop Wearable System to Reduce Nasal Injuries in Preterm Infants |
|
|
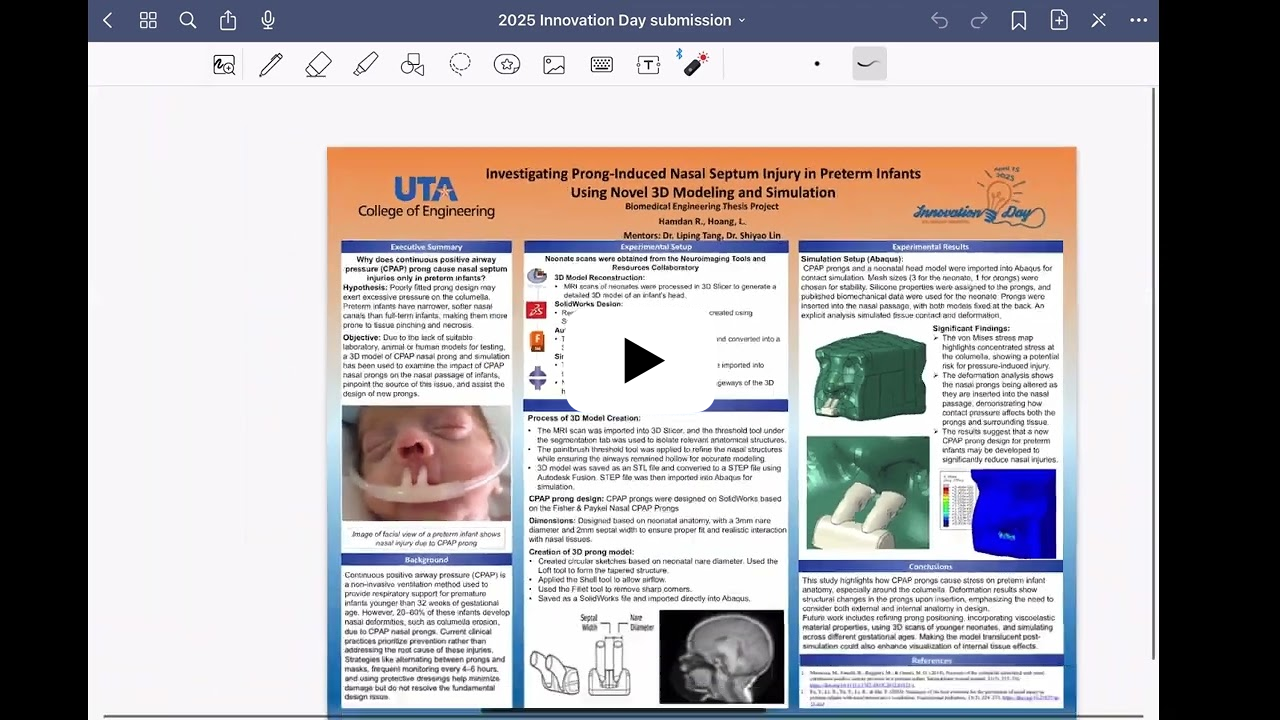
Prolonged use of CPAP nasal prongs in preterm infants with respiratory distress syndrome can cause septal damage, columella necrosis, and long-term nasal deformities.
Researchers at UT Arlington Research Institute (UTARI) are developing a wearable sensor and digital twinning (WSDT) system to monitor and mitigate nasal-prong-induced stresses. This low-cost, reusable system aims to guide clinical decision-making, improve nasal prong design, and reduce the risk of nasal injuries in neonates. |
|
UT Dallas Launches AIM S&E Program to Support STEM Master’s Students |
|
|
The University of Texas at Dallas has launched the Advancing Innovation in Master of Science and Engineering (AIM S&E) Scholars Career Development program, providing financial, academic, and career support to low-income students pursuing master’s degrees in STEM fields.
Funded by the NSF S-STEM program, the initiative offers scholarships and connects students with faculty and industry mentors through transdisciplinary mentoring pods. Participants gain guidance, networking, and career development to pursue PhDs or enter high-demand STEM fields such as AI, biomedical sciences, bioengineering, and data science. |
|
UT Southwestern and Children’s Health Advance Pediatric Care with New Campus |
|
|
|
UT Southwestern Medical Center and Children’s Health℠ are developing a transformative joint pediatric campus in Dallas’ Southwestern Medical District. The Specialty Center on the new campus will feature the Harry W. Bass, Jr. Foundation Lobby.
The campus, which broke ground in October 2024, will include Moody Children’s Hospital with two 12-story towers and one eight-story tower, significantly expanding inpatient, surgical, and ambulatory care. It is expected to open in 2031. |
|
EVENT RECAP |
|
Life Science Oklahoma Kicked Off Its Annual Catalyst Series |
|
|
BioNTX was honored to attend Life Science Oklahoma’s inaugural Catalyst Series luncheon, featuring Kathy VanEnkevort, Microsoft’s U.S. Health & Life Sciences Leader.
|
|
|
This event also marks an important step in BioNTX’s growing engagement with neighboring states, as we work to strengthen cross-border collaboration, knowledge exchange, and shared opportunities across the region’s expanding life science ecosystem. |
|
Finding Clarity in the Chaos: Insights from Ignite’s Fireside Chat on Healthcare Innovation |
|
|
For industry leaders navigating a year defined by funding cuts, policy shifts, and a cooling investment climate, Ignite’s recent fireside chat offered a timely dose of perspective and optimism.
The conversation brought together entrepreneurs and investors on the front lines of healthcare innovation to share how they are adapting, what they have learned, and where they see opportunities emerging.
Amid volatility and uncertainty, key themes stood out—collaboration, creativity, and resilience remain essential drivers of progress. The dialogue underscored that while the path forward may be shifting, the momentum behind healthcare innovation is as strong as ever. |
|
|
(left to right) Sandra Saldana, CEO & Co-Founder, Alva Health; James Wang, Founder, Mimosa Ventures; Kim Schwartz, Managing Director, Accenture; Emily Coskey, Associate, Wilson Sonsini; Ayse McCracken, Founder of Ignite Healthcare |
|
FUNDING & MENTORSHIP OPPORTUNITIES |
|
Nucleate Activator Program Application closes October 15, 2025 |
|
|
Are you a scientific trainee (PhD, Post-doc, MBA, MD, JD) interested in launching a biotech or sustainability-focused startup? Are you working on innovations in therapeutics, diagnostics, medtech, synthetic biology, or tackling challenges in food, energy, agriculture, or consumer products?
|
|
Advancing Therapeutics Reprogramming for Military Regenerative Medicine Application closes October 17, 2025 |
|
|
This solicitation is issued by the Wake Forest Institute for Regenerative Medicine (WFIRM) as the AFIRM Coordinating Center (CC).
This Request for Project Proposals (RPP) is focused on supporting regenerative medicine research to improve prevention, detection, diagnosis, treatment, and/or quality of life. Awards made from this effort are intended to support translational research or clinical trials. |
|
ARPA-H Give Application closes October 19, 2025 |
|
|
GIVE is aimed at creating distributed biomanufacturing and quality control platforms for genetic medicines and cell therapies to enable onsite production and access. Projects should focus on overcoming key challenges to manufacturing genetic medicines including manufacturing costs, the reliance on a discrete subset of locations that provide unique centralized equipment, resource intensive quality control systems, and the need for ultracold storage and shipping of finished products.
Through this program, ARPA-H aims to bolster U.S. biomanufacturing technology to ensure personalized and individualized genetic medicines are made domestically, improving patient access to cancer therapies, cell therapies, and gene editing technologies. Applicants should ensure strong expertise in automation, device integration, and quality systems to compete in this infrastructure-oriented space. |
|
ARPA-H THRIVE Application closes October 31, 2025 |
|
|
THRIVE aims to make personalized and affordable cures available to all rare disease patients. The program intends to develop pioneering integrated platform technologies to accelerate precision genetic medicines (PGMs) and provide single-intervention precision treatments to slow, reverse, or prevent chronic diseases at the genetic level. THRIVE is designed to optimize affordability, scalability, and sustainability of lifesaving PGMs for patients through existing regional treatment centers and virtual clinics. This will allow patients to be seen and treated where they live. |
|
CPRIT High-Impact/ High-Risk Research Awards Application closes December 2, 2025 |
|
|
The High-Impact/High-Risk Research Award (HIHRA) supports research that explore the feasibility of high-risk projects that, if successful, would contribute to major new insights into the etiology, diagnosis, treatment, or prevention of cancers.
Using this mechanism, the Cancer Prevention and Research Institute of Texas (CPRIT) intends to support innovative, developmental projects that focus on exceptionally promising topics that are not yet sufficiently mature to compete successfully for more conventional funding.
The HIHRA is expected to provide the foundation and preliminary data for individual or multiple investigator peer-reviewed awards. |
|
Fueling the Next Generation of Health Innovators Application closes March 6, 2026 |
|
|
The One Health Incubator is a dynamic partnership between the Innovation Hub and the Health Sciences Center, created to support researchers and technology startups in the life sciences sector. This collaborative platform combines institutional expertise to offer an ecosystem of incubation, mentorship, and strategic connections—tailored specifically for life science innovators.
Startups and researchers benefit from access to state-of-the-art facilities, specialized resources, and guidance from industry experts. The incubator helps accelerate the development and commercialization of innovative healthcare solutions while fostering valuable relationships with potential partners, investors, and mentors. |
|
NIH Academic Research Enhancement Award (AREA) for Undergraduate-Focused Institutions Program ends January 8, 2027 |
|
|
The purpose of this Academic Research Enhancement Award (AREA) for Undergraduate-Focused Institutions is to support small scale research grants at institutions that do not receive substantial funding from the NIH, with an emphasis on providing biomedical research experiences primarily for undergraduate students, and enhancing the research environment at applicant institutions. |
|
INDUSTRY EVENTS |
|
Upcoming Events & Opportunities |
|
|
Presents
BIO BREAK Fort Worth - Oktoberfest
Join BioNTX, RSM, and UMB Bank for a special Oktoberfest-themed happy hour at Boehringer Ingelheim, celebrating the life science community where ideas flourish, partnerships take root, and collaboration thrives.
October 14th | Fort Worth, TX |
|
|
Presents
So You Think You Need a Facility?
Join the BioNTX Facilities Committee to hear a discussion on guiding start-ups from research to production. We will cover options such as co-working spaces like BioLabs and Bridge Labs, partnering with CDMOs, or building your own facility from scratch or through renovations.
October 15th | Dallas, TX |
|
|
CRISPR Conversations: Ensuring Quality, Success, and Scale with Guide RNA
Join TriLink BioTechnologies to explore strategies for optimizing guide RNA quality, improving editing success, and scaling for translational and clinical applications. It is a great opportunity to connect with gene editing innovators and join the conversation on where the field is headed.
October 15th | Houston, TX |
|
|
IND 101
Join NCTM for an interactive workshop designed for research, medical, and biotech professionals who need a practical understanding of the Investigational New Drug (IND) process. Topics include preparing for and conducting a pre-IND meeting, IND format and content, and leveraging FDA’s expedited pathways.
October 23rd - 24th | Hybrid |
|
Thank you for your Membership & Support! "The greatest wealth is health." - Virgil |
|
|
October 13, 2025 |